Lab 12: The Ozone Hole and the Smog
Part One- Stratospheric Ozone
Refer to the figure below and text to answer the following questions.
1. What comprises the ozone molecule?
2. What is the importance of the ozone layer in the stratosphere and what consequences would occur if it did not exist?
3. Which element in CFC’s breakdown ozone molecules?
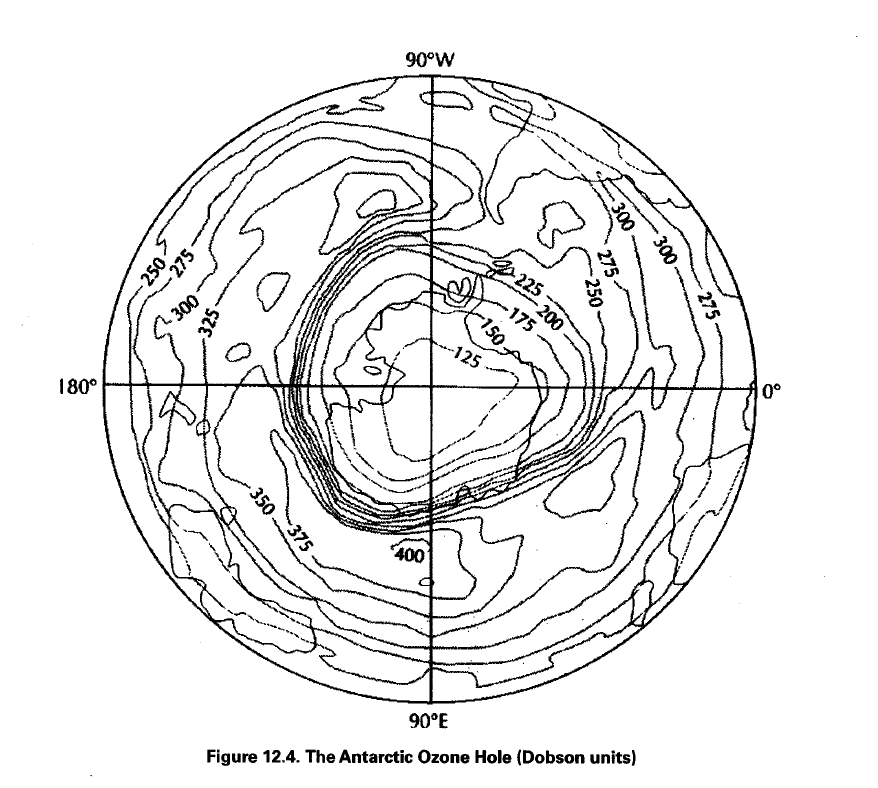
4. Where is the maximum depletion of the ozone hole occurring (Fig. 12.4)?
5. During which months is the ozone hole over Antarctica present?
6. What is a Dobson unit?
7. How many Dobson units are there at the region of maximum depletion (Fig. 12.4)?

Part 2- Tropospheric Ozone
The stratospheric ozone is well known as an important gas to protect surface from the solar UV radiation. The depletion of the stratospheric ozone is called " ozone hole" and it is a serious problem for the globe. The tropospheric ozone is important as well as the stratospheric ozone and its dynamic and chemical behaviors are more complicated and have not well known yet.
The characters of the tropospheric ozone
About 8 % of the total column ozone is in the troposphere. The tropospheric
ozone also plays an important role in the atmosphere.
1) Ozone is a green house gas and possibly contributes to the global warming.
2) Ozone is harmful for human being and crops in the troposphere.
3) Ozone oxidizes many chemical substances in the troposphere, and controls
tropospheric chemistry.
The source of the tropospheric ozone.
The tropospheric ozone has two major sources. One is intrusion from the stratosphere. Most of ozone in the atmosphere is in the stratosphere and created from solar UV radiation. The stratospheric ozone sometimes flows into the troposphere by the upper layer trough and cut-off low activities. Some part of ozone may subside in the troposphere directly by the Hadley circulation or the Brewer Dobson circulation.
Another source is production from photochemical reactions. Ozone is produced from relatively longer-wavelength solar radiation under NOx and non-methane hydrocarbons rich environments. These photochemical reactions possibly bring about photochemical smog in urban area.
The trend of the troposheric ozone
Some global monitoring stations show the increase of ozone in the
troposphere. It may be a big problem, because if tropospheric ozone increases,
it might damage to human beings and many crops, and fairly contribute to the
global warming and change the tropospheric chemistry. If the tropospheric
chemistry changes, the nature of pollution and acid rain might change.
But not all monitoring stations show the increasing trend. We have to monitor
the tropospheric ozone at many suitable observation points. This also has the
mean to study influence of human activities on Nature.
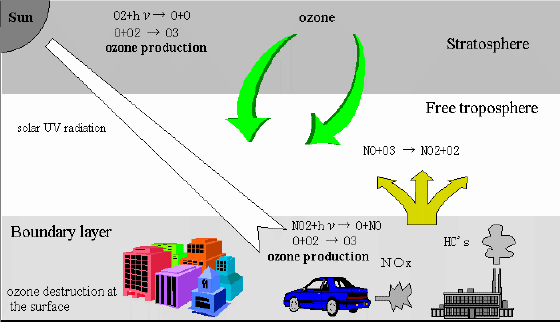
1. Why is tropospheric ozone considered "bad" ozone?
2. What are the damaging effects of surface level ozone?
3. What are two sources of ground level ozone?
4. When is tropospheric ozone most often encountered and why?
5. What is an Ozone Action Day?
6. What are three things you can do on an Ozone Action Day to reduce the buildup of ozone in the troposphere?