DIAMOND CRYSTAL STRUCTURE |
|
 |
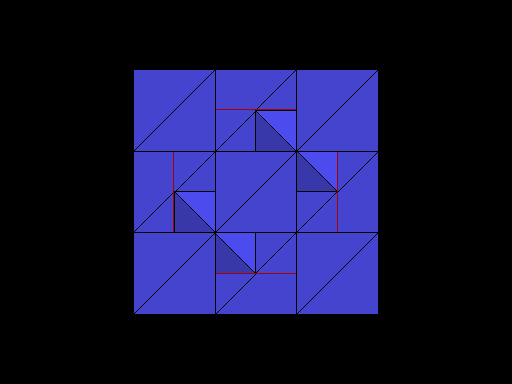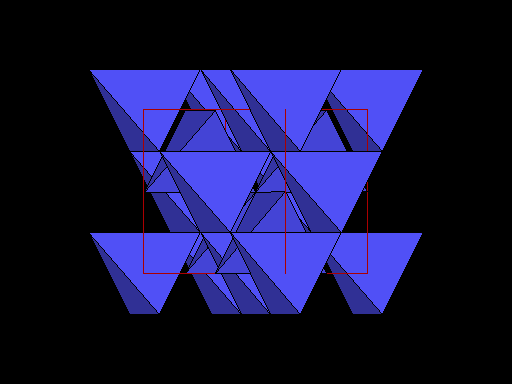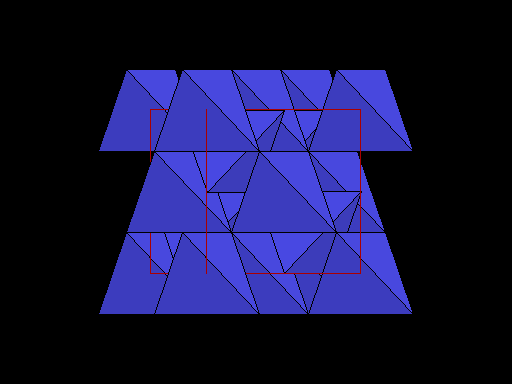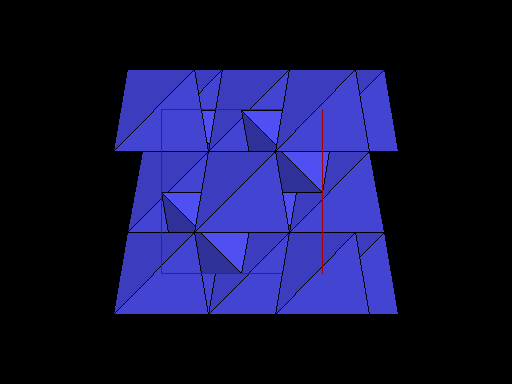 |
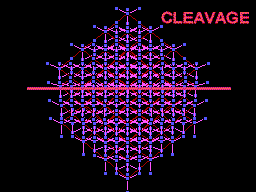
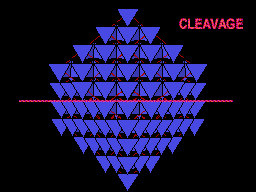
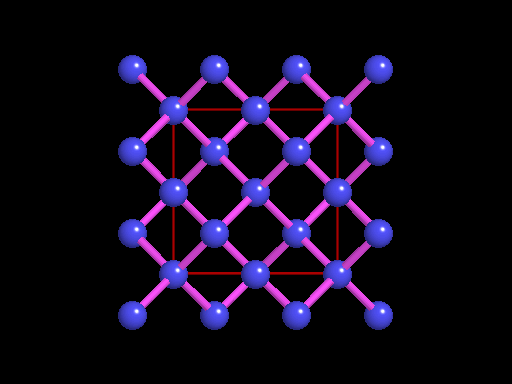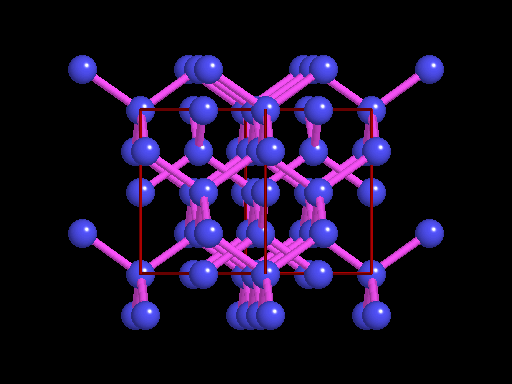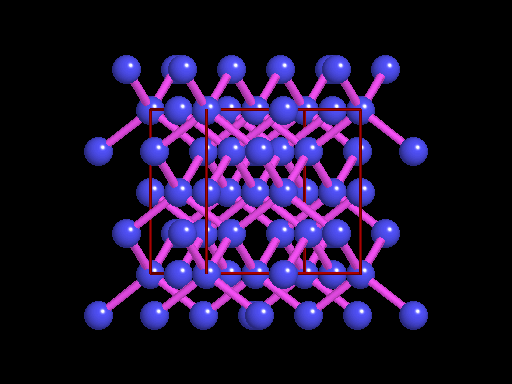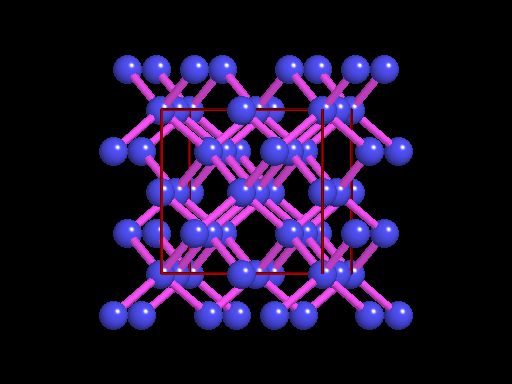
The unit cell (red) of diamond is a cube. Groups of five carbon atoms (blue spheres) form tetrahedra at the cube corners, at the centers of each of the cube faces, and at four sites within the cube. This is shown best in the view on the right, a polyhedral model showing the tetrahedra rather than the atoms. The carbon atoms are located at the center and four apices of each tetrahedron. The carbon tetrahedra are covalently bonded, accounting for diamond's extreme hardness. The toughness of diamond is compromised somewhat by the presence of 4 planar directions of weakness, its octahedral cleavage. These cleavage directions are at 45o to the corners of the cube. |
|
|
|
Diamond oriented so that a cleavage plane (pink line) is horizontal. |
Same view but with carbon atoms shown as tetrahedra to better show this plane of weakness. Note the prominent sheets of carbon atoms parallel to the cleavage. |